The monkey we now have news of is genetically closer to humans than macaques, which raises fears that reproductive cloning could be completed in humans in a not excessively long period.
Although some Chinese companies have already stated their intention to clone human beings for reproductive purposes, the technical difficulties in achieving this are insurmountable today. But the dizzying progress of genetic editing techniques and the deeper knowledge of the functioning of our genome and the epigenetic mechanisms on which the development of the embryo depends makes us fear that reproductive cloning in humans could be a reality in not long time.
A new cloning experiment, conducted in China in 2022 and described in Nature Communications, marks the first successful cloning of the species and was achieved using a slightly different approach to the conventional cloning technique used to clone Dolly the sheep, and other mammals, including the long-tailed macaques (Macaca fascicularis), which were the first primates to be cloned.
The experiment, which has just been made public, involves obtaining a rhesus monkey primate that is genetically very close to the human species, after a somatic cell nuclear transfer (SCNT) experiment.
Previously, in 2018, the birth of a macaque (Macaca fascicularis) also generated by cloning was reported, although the current case presents differences that we explain later.
What is cloning?
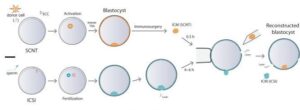
Somatic cell nuclear transfer (SCNT) technology consists of producing individuals of one species whose genome comes from an adult cell of another, being introduced into a previously enucleated oocyte. In this way, genetically identical individuals are obtained.
This technique has been widely used for cloning several species of mammals, including sheep, cows, mice, pigs, goats, rabbits and dogs. The aforementioned case of macaque cloning in 2018 has represented an important qualitative change in reproductive cloning attempts, given the genetic complexity of primates and their proximity to humans. These difficulties, until then insurmountable, were overcome by applying epigenetic regulators in the preimplantation process. This allowed the expression of certain genes to be regulated, finally allowing the birth of the first cloned primate. During this previous macaque cloning experiment, 109 cloned embryos were created, with almost three-quarters of them implanted into 21 surrogate monkeys, resulting in six pregnancies. Only two of the monkeys survived birth.
In humans, therapeutic, non-reproductive cloning has been carried out with the intention of obtaining stem cells from cloned embryos – stem cells – that can be used in regenerative medicine. That is, obtaining cloned embryos from an individual from which stem cells would be extracted that, properly manipulated, could contribute to the regeneration of organs or tissues of that same individual, thus eliminating the problems associated with the immunological rejection processes associated with transplants. .
Technique efficiency
The live birth rate in the case of previous cloning of macaques was 2% on average. In conventional cloning methods, live birth rates for most mammalian species are between 1% and 3%, with slightly higher rates observed for cattle (5%-20%). Furthermore, it should be noted that developmental anomalies occur, particularly in extraembryonic lineages, such as placental tissues.
In the recent case of the experiment with rhesus monkeys, the birth of a single live cloned specimen was achieved from 113 initial embryos, which implies an efficiency of less than 1%.
One more step
The study, now published, has investigated the genetic differences of embryos obtained by in vitro fertilization, specifically through the intracytoplasmic sperm injection (ICSI) technique, with others obtained through cloning processes (SCNT). The researchers found important differences in the epigenetics of both groups, consisting of a general decrease in DNA methylation and the loss of maternal imprinting in the genes of the cloned embryos.
These changes in epigenetic information translate into histological modifications in the placentas of cloned embryos, which show notable hyperplasia and calcification.
Those responsible for the study devised a novel procedure to “replace” the placental tissues of the cloned embryos with those obtained from the non-cloned ones resulting from in vitro fertilization techniques, specifically ICSI. They devised a procedure called “trophoblast replacement method” (ReTro), in which they combined cells from both types of embryo: those that would develop the fetus came from the cloned embryo and those that would give rise to the placenta, from the non-cloned one, ultimately leading to the birth of a healthy male rhesus monkey that has survived for more than 2 years at the time of preparing this publication. The procedure consisted of the injection of the inner cell mass (ICM) derived from cloned embryos (SCNT) into the blastoceles (without the ICM) derived from embryos produced with intracytoplasmic sperm injection (ICSI).
The birth of a cloned rhesus monkey with somatic cells by trophoblast replacement
Liao, Z., Zhang, J., Sun, S. et al. Reprogramming mechanism dissection and trophoblast replacement application in monkey somatic cell nuclear transfer. Nat Commun 15, 5 (2024).
Consequences of this new discovery
These findings provide valuable information on the mechanism of genetic reprogramming that occurs after cloning of monkeys and open the way to the extension of primate cloning, offering new possibilities to the alarming possibility of reproductive cloning in humans.
Mu-ming Poo, director of the Institute of Neuroscience at the Chinese Academy of Sciences in Shanghai, said: “We can produce large numbers of genetically identical monkeys that can be used for drug efficacy testing,” eliminating differences in responses attributable to the genetic variability of the study specimens.
Bioethical assessment
Obtaining individuals of a species through the technique of nuclear transfer of somatic cells (cloning) whose genome is an identical copy of that of another individual, constitutes an alternative that violates the natural procedure by which living beings that use sexual reproduction perpetuate their species. . Through sexual reproduction, nature has ensured genetic variability, causing offspring to inherit the genetic material of their parents, combined in an original and unrepeatable way. This genetic variability is what ensures the evolution of the species we know today. On the other hand, cloning techniques for reproductive purposes, such as the one used in the recently published case of the rhesus monkey, have a low success rate, which requires the creation of numerous embryos and the use of many pregnant females, and in most cases the process fails due to the genetic anomalies that accumulate during the manipulation to which these embryos are subjected. In conventional cloning methods, live birth rates for most mammalian species are extremely low, ranging between 1% and 3%, with slightly higher rates observed for cattle (5%-20%). This involves enormous efforts with poor results and the loss of numerous embryos in the process. As already mentioned, the birth of a macaque in 2018 was a first step in the race towards overcoming the difficulties of achieving the birth of a live cloned human being.
The monkey we now have news of is genetically closer to humans than macaques, which raises fears that reproductive cloning could be completed in humans in a not excessively long period of time. Obtaining cloned human individuals through manipulation offers ethical difficulties that are difficult to overcome, since it implies the genetic predesign of the individual and the possibilities of its instrumentalization, which represent a violation of the dignity of the individual as a unique and unrepeatable human being.
Currently, unlike what happens in China, reproductive cloning experiments with primates are not permitted in the European Union, due to their genetic proximity to the human species, unless the experiment is aimed at investigating a serious, fatal disease. , which affects human beings or the primate species itself, which is not the case in this experiment.
But it must be clarified that the cloning of human beings has been carried out for more than a decade, beginning with the experiences of Mitalipov, but not with reproductive intentions but with research intentions. The laws of the countries that regulate this type of tests allow human embryos to be obtained by cloning to be used in research, but with limitations, and they must be destroyed at an early stage in their evolution, when they contain up to 200 cells. These early embryos are also individuals of the human species, artificially produced to be destroyed. It is not easy to justify it ethically either, especially when the number of embryos generated and destroyed is enormous, and the results are scarce.
The clinical application of regenerative therapies based on stem cells obtained from cloned embryos is very far from being a reality in medicine. The current development of research into therapies with induced pluripotent cells, known as iPS, makes unnecessary the costly and ineffective cloning process and the bioethically unacceptable creation and destruction of human embryos that it entails.
Although some Chinese companies have already stated their intention to clone human beings for reproductive purposes, the technical difficulties in achieving this are insurmountable today. But the dizzying progress of genetic editing techniques and the deeper knowledge of the functioning of our genome and the epigenetic mechanisms on which the development of the embryo depends makes us fear that reproductive cloning in humans could be a reality in the not long time.
Not everything that can be done in research should be done. Reversing sexual reproduction through the use of cloning techniques does not constitute an evolutionary advance, but rather a regression. Utilitarian scientists for whom the end justifies the means frequently ignore that some ends presented as scientific advances are not actually such, but rather setbacks. A rigorous analysis of the consequences of these experiments, their usefulness and their risks, must inspire the necessary regulation of these research practices, in defense of our species, our ecosystem and our dignity as people.
Julio Tudela – Bioethics Observatory – Life Sciences Institute – Catholic University of Valencia